What Aduhelm’s Trial Got Right (That No One’s Talking About)
Aduhelm's FDA approval was messy. But behind the scenes, operations held strong. Here’s what clinical trial sponsors can learn from Biogen's operations.
RJ Gumban
5/6/20256 min read
What happens when the first major breakthrough for Alzheimer’s becomes one of the most divisive FDA approvals in recent memory?
In 2021, Aduhelm (aducanumab) made headlines as the first Alzheimer’s treatment approved based on amyloid plaque reduction rather than proven clinical benefit. For a moment, it felt like the future had arrived.
Then came the fallout.
One trial showed benefit. Another did not. Advisory panel members resigned. Hospitals refused to administer it. Medicare limited coverage to patients in clinical trials.
A historic decision quickly spiraled into a firestorm of regulatory and public scrutiny.
At Freelance Clinical, we look beyond the controversy. Underneath it all, Aduhelm’s operations reveal lessons every clinical trial sponsor should study. Especially when the science gets complicated.
When the data is uncertain, execution becomes the difference between collapse and continuation.
Why Aduhelm sparked a firestorm
Aduhelm (aducanumab) was positioned as the first real breakthrough for Alzheimer’s disease. In 2021, it became the first treatment approved to target the buildup of amyloid plaques, a hallmark of the disease.


It felt historic. Then everything unraveled.
Biogen ran two near-identical Phase 3 trials: EMERGE, which showed a modest slowing of cognitive decline, and ENGAGE, which showed no benefit at all.
The program was halted for futility and then reanalyzed. Biogen argued that a higher-dose subgroup in EMERGE supported efficacy.
This post hoc interpretation sparked sharp criticism. Many in the scientific community questioned whether the positive signal was real or just statistical noise.
Regulatory decisions added more fuel. The FDA advisory panel voted overwhelmingly against approval. Still, the agency moved forward. Their decision focused on plaque reduction, not clinical outcomes. Several panelists resigned in protest, citing review concerns and a lack of adherence to good clinical practice.
What Biogen got right behind the scenes
Even though the clinical results faced scrutiny, Aduhelm’s execution delivered outcomes worth examining on their own. The ability to scale, adapt, and coordinate across a global footprint helped keep the program alive when many others would have stalled.
Here is what the Aduhelm trials got right.
Global patient enrollment at scale
Finding patients with early-stage Alzheimer’s is already challenging. Requiring amyloid PET scans for confirmation raised the bar even higher.
Yet Biogen enrolled over 3,200 patients across 348 sites in 20 countries, creating one of the largest and most complex Alzheimer’s trial networks to date.
They succeeded by strengthening referral pathways, supporting investigators with patient identification tools, and anticipating recruitment bottlenecks early. These are the kinds of clinical trial solutions sponsors often overlook until timelines start slipping.
For any clinical trial sponsor, building global enrollment capacity like this reflects a proactive and thoughtful clinical operations strategy.
Operational agility and adaptation
Midway through the trials, an interim analysis suggested limited success, so Biogen made the difficult decision to halt them.
When additional data emerged, they moved quickly to reanalyze results, interpret new findings, and reengage trial sites and participants.
Pivoting a global Alzheimer’s trial after a public futility halt is exceptionally rare. Recovery required more than updated data. Trust at every level of the trial network depended on rapid coordination and clear communication.
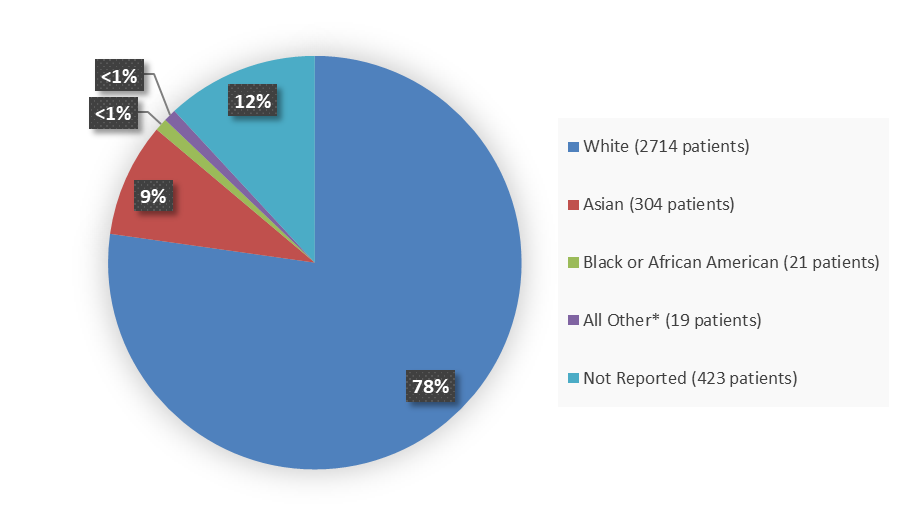
Leveraging external operational partners
Biogen’s internal team was supported by extensive partnerships in clinical trials, including a strategic collaboration with Quintiles (now IQVIA).
Experienced external teams managed critical functions like site monitoring, data management, and imaging reviews. This allowed Biogen to scale operations across 20 countries while keeping standards consistent.
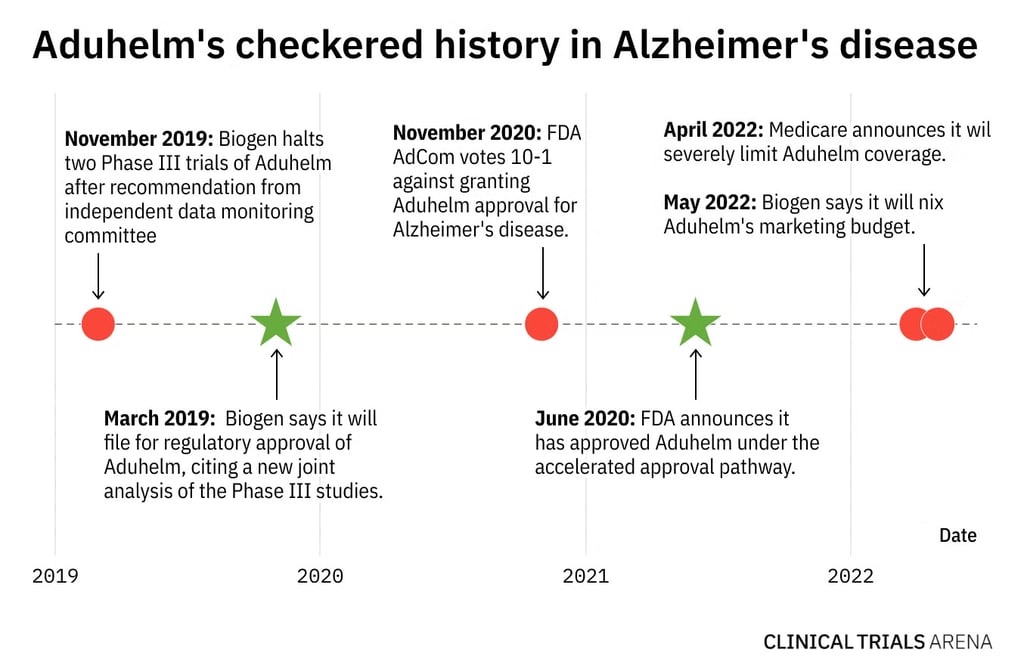
5 sponsor lessons buried in the Aduhelm trial
Aduhelm’s operational journey wasn’t perfect. But it showed how large-scale trials can remain stable under pressure.
The following lessons offer practical strategies that researchers and clinical trial sponsors can use to manage complexity with more control and confidence, especially in later-stage programs where timelines tighten, and expectations grow.
1. Prioritize patient pathways early
Enrollment delays rarely begin at site activation. Most start long before that.
Uncoordinated referrals, ambiguous eligibility criteria, and unsupported sites often lead to poor screening conversion. Trials involving high failure rates in clinical trial phases, such as early-stage Alzheimer’s, require tighter planning.
Sponsors should align imaging timelines, central screening processes, and referral workflows well before the first patient arrives. Removing friction between diagnosis and PET-confirmed eligibility helps maintain steady enrollment.
2. Build operational flexibility into study design
Trial data does not always follow protocol assumptions. Rigid study designs often prevent teams from responding when early trends shift.
Sponsors should design with optionality. That means including interim analyses with clear thresholds, protocol language that allows dose adjustments, and data systems that can handle amendments without disrupting operations.


Biogen headquarters on Binney Street in Cambridge. (Jesse Costa/WBUR)
Source: Clinical Trials Arena
That level of agility is what separates resilient clinical operations strategy from reactive trial management.
The public reaction followed quickly:
Major hospitals like Cleveland Clinic and Mount Sinai declined to offer the drug.
Medicare limited coverage to patients enrolled in clinical trials.
The original price of $56,000 per year drew backlash and was later reduced by 50 percent.
For any clinical trial sponsor, Aduhelm’s story became more than a regulatory outlier. It became a case study of how evidence, communication, and credibility can collide under public and scientific pressure.
Source: Adapted from FDA Review
These outsourced resources weren’t just there to extend bandwidth. They helped standardize procedures, safeguard data integrity, and maintain operational quality across varied regulatory environments.
For sponsors without in-house scale, these kinds of collaborations are essential to strong execution.


Source: Adapted from IQVIA
Biogen’s ability to reassess and restart after its initial futility decision was only possible because of operational flexibility already built into the structure.
3. Strengthen site communication and trust
Sites are often the first to feel disruptions. If investigators lack timely updates or a direct point of contact, engagement drops quickly.
Sponsors should establish clear, repeatable communication systems. This might include real-time dashboards, alert tools, or accessible support teams.
Trust is not built during launch meetings. It's earned through consistency, especially when the trial's direction becomes uncertain.
For many programs, that trust is delivered through the clinical research associates who maintain direct site relationships.
4. Secure external expertise before scaling
Global trials amplify even small missteps. A delayed SOP rollout or inconsistent imaging process can cause widespread setbacks when sites are spread across multiple regions.
That is why experienced external partners should be engaged early. These teams support monitoring, data review, safety oversight, and documentation without draining internal capacity.
Sponsors without built-in infrastructure benefit most from outside help. Many now rely on freelance clinical research experts to provide fast, targeted support without long onboarding cycles.


5. Prepare for post hoc data strategies carefully
When primary outcomes fall short, exploratory data may offer new directions. But only if the framework for reanalysis already exists.
Sponsors should anticipate this possibility by building imaging repositories, identifying exploratory endpoints in advance, and setting up processes for validated sensitivity testing.
Biogen’s post-hoc pivot was not made up on the spot. It was supported by documentation, planning, and tools that made additional reviews credible.
Only 1.8% of Alzheimer’s drugs that enter Phase 3 make it to market. Aduhelm did, but not without a storm.
Operational strength still matters
Aduhelm’s story reminds us that clinical trials don’t succeed or fail on results alone. How those results are generated and how teams respond throughout the process matters just as much.
Strong operations do more than keep a study moving. They create the foundation for wise decisions, timely pivots, and long-term credibility.
Without that structure, even promising therapies can lose momentum.
For every clinical trial sponsor planning a complex study, the message is clear: build a team that can handle uncertainty. Design systems that evolve with the data. Prioritize operational readiness from the start.
Freelance Clinical supports sponsors with experienced professionals and tailored strategies across every execution stage. If you need flexible support, staffing, or guidance through your next trial, our freelance clinical research network is here to help.
Submit a request through our form, and we’ll match you with the right experts to support your upcoming study.
Disclaimer
This article is for informational purposes only and does not constitute legal, regulatory, or medical advice. The views expressed are those of the author(s) and do not reflect official positions of any organizations referenced. Mentions of Aduhelm, Biogen, or any third-party entities are based on publicly available information and cited sources. Clinical trial sponsors should consult appropriate experts and regulatory bodies before making decisions based on the topics discussed.
FOLLOW US
Disclaimer: Freelance Clinical provides freelancer and vendor sourcing services for clinical research support. We do not claim to list or compare all available providers in the market. All information and recommendations are general in nature and do not constitute endorsements or guarantees. Clients are responsible for conducting their own due diligence before engaging third parties. Freelance Clinical is not liable for any outcomes resulting from such engagements. This website is governed by the laws of New South Wales, Australia.
ABN: 12 671 763 643 · © 2025 Freelance Clinical Pty Ltd. All rights reserved.
