How Clinical Vendors Get Shortlisted by Sponsors
Learn how clinical vendors get shortlisted. Show fit, proof, and readiness fast to move from skim to call, and from call to award.
RJ Gumban
9/8/20255 min read
Global trial teams scan vendor options fast. Profiles get seconds, not minutes. Too many listings bury value, skip proof, and leave sponsors guessing about readiness and coverage.
Data echoes this: clinical research associate (CRA) turnover sits near the low 20% and protocol amendments keep rising.
As a result, sponsors slog through long PDFs and vague pages, add calls to fill gaps, and delay awards while they chase basics. Timelines also slip not because vendors lack capability, but because buyers cannot see fit or trust the promise quickly.
From our reviews of shortlists and awards, a few simple changes consistently move vendors from view to call. Below are the ones that work.
Make fit obvious at first glance
Shortlisting starts with a message match. Your first screen should answer a sponsor’s first questions without scrolling. Keep it crisp and specific.


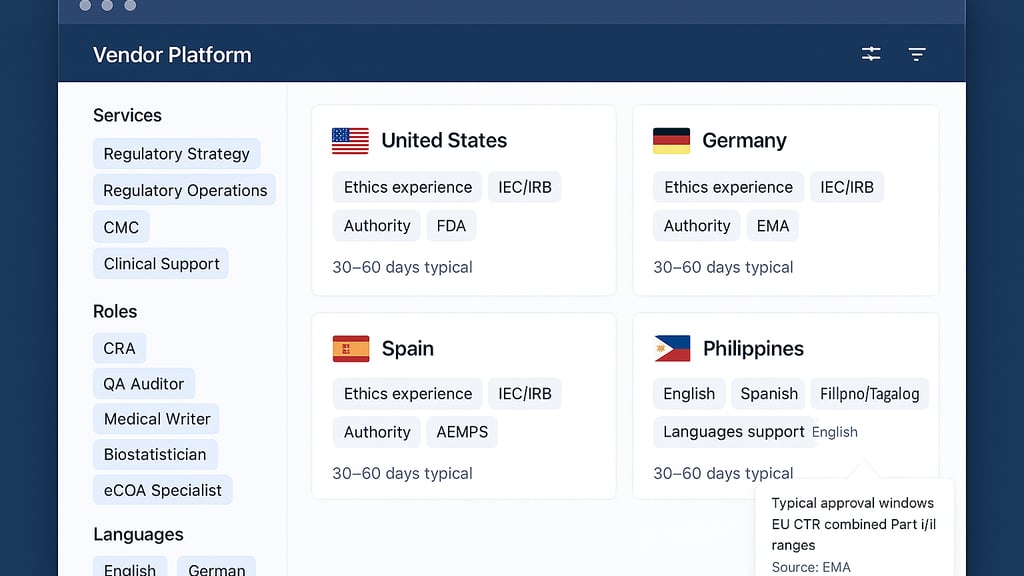
Use sponsor language for services. Replace internal jargon with terms buyers search for. See how ICON’s regulatory services taxonomy groups strategy, operations, CMC, and clinical support, making it clear in seconds.
Put markets and languages up front. Create country cards that showcase expertise in ethics, authority, and language support, as well as activation context. For structure, mirror our Freelance Talent Sourcing page, which lists common roles and the countries our freelancers serve.
Add realistic timing cues. When you mention typical approval windows, anchor them with a credible source so ranges do not feel vague. Accelsiors outlines EU clinical trial timelines for combined Part I and Part II submissions you can reference.
Show you are ready to move timelines
Sponsors shortlist partners who can start on time and keep momentum when issues arise. Readiness is not a slogan. It’s something you show. A simple one-page checklist signals that your team is organized and launch-ready.
Here’s one straightforward example we found: the Implementation Readiness Checklist. It lists milestones with a simple “completed” column, such as recruitment plans finalized, ethics and regulatory oversight confirmed, site training ready, data collection methods tested, and budget validated.
Use this as inspiration, not a rule. Keep your version brief, date it, and update weekly. Put your start window, country capacity, and named delivery lead with deputy at the top.
Attach the sheet to proposals and your profile so sponsors can see at a glance that you are prepared to begin and able to keep timelines moving.
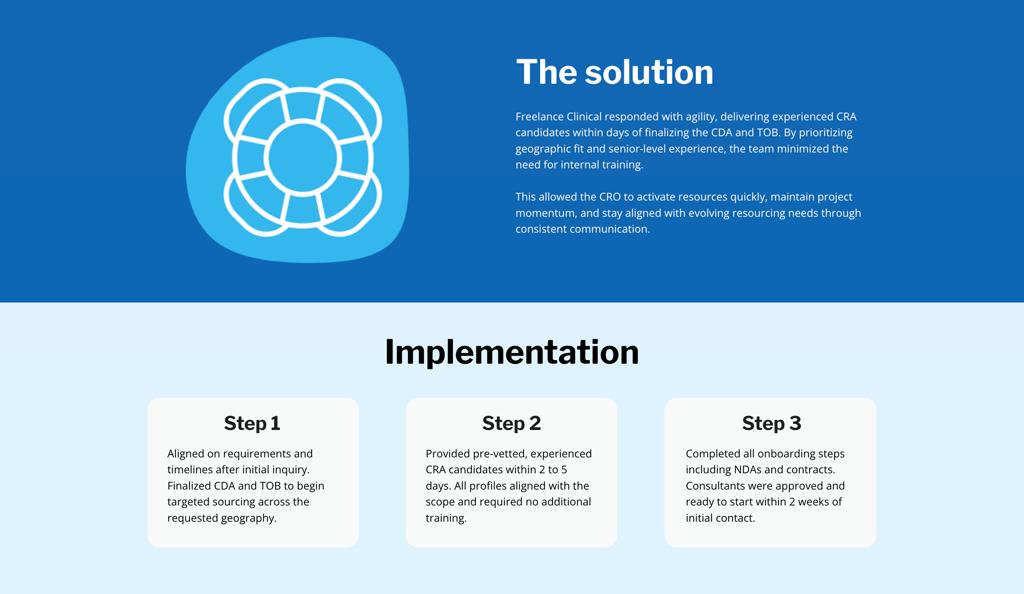
Signal how you partner and communicate
Treat this like coaching for your future team. Competence gets you to the door, and communication keeps you in the room. Make your working model explicit. In intake and kickoff, define roles, decision rights, and escalation paths.
You can anchor this with real operating principles and partnership examples, such as Suvoda’s guidance on forging strong sponsor–vendor partnerships and their case library.
Next, set a week-one cadence that prevents drift: daily check-ins for launch week, a named owner for issues, and a simple change-control path that spells out who decides, what inputs are needed, and turnaround targets. WCG’s practical notes on start-up delays map well to these behaviors and reinforce process clarity.
Finally, commit to a reporting rhythm with metrics that matter to sponsors. Share a one-page dashboard that includes query aging, referral yield, and consent-to-randomization conversion, mirroring how OneStudyTeam frames recruitment analytics and diversity reporting.
Ready to be shortlisted more often?
Strong vendor profiles do the simple things well. Lead with clear services and markets, show proof that you reduce risk, publish a one-page readiness checklist, set straightforward pricing expectations, and make your working rhythm easy to understand.
These small shifts move you from a quick skim to a call, and from a call to an award.
If you want a quick, practical lift, we can help. By listing through our services, more sponsors will see your capabilities, country coverage, and proof points. Start here: Global Vendor Sourcing.
We are opening early access to our vendor platform that helps sponsors find qualified vendors faster. Join the early list here.
Tune up your profile now, get discoverable next, and make the shortlist first!
Prove you reduce risk
Fit earns attention, and proof earns trust. Sponsors look for signals they can verify and outcomes that show you keep timelines intact. Start with one clear line on quality covering standard operating procedures (SOPs), good clinical practice (GCP) training, and corrective and preventive action (CAPA). Then show outcomes, not adjectives.
Pro tip: Mirror sponsor filters. Use the same service labels and country names across your site, LinkedIn, and materials. Consistency makes you easier to match and prevents being overlooked.
See how Worldwide Clinical Trials kept an NDA on schedule using a clear, time-stamped timeline; how our Fenix Biosciences case shows the problem, challenges, solution, 3-step implementation, and impact; and how Medpace beat database lock targets with a defined plan and weekly metrics
Make it easy to check your claims:
Two short case stories with one metric each
A cadence signal, such as weekly listings or query aging under target
Reference checks on request with a brief engagement outline
Want these proof points seen? Vendors can join the early list for our vendor platform here, and sponsors who need a vetted shortlist can start with Global Vendor Sourcing.
Set pricing expectations early
Sponsors don’t expect a public rate card to fit every scenario. But they do expect enough detail to gauge scope fit before a call. Set a simple, repeatable structure and borrow cues from clean pricing models and milestone packages.
Define your pricing model. State when you use milestones, packaged fees, or hourly support, with one-line examples: first-country ethics submission, eCOA build and user acceptance testing (UAT), site-feasibility bundle. For data work, outline milestones tied to database build, interim outputs, and database lock.
List key assumptions. Spell out what is included, what triggers a change order, inputs required to begin, and acceptance criteria for each milestone. This reduces procurement back-and-forth and avoids surprise overruns.
Show a simple timeline. A short bar across intake, planning, execution, and closeout shows you understand dependencies. If you support analysis, note when the statistical analysis plan (SAP) locks and how you coordinate clinical study report (CSR) production.
Pro tip: Create two or three standard packages with clean assumptions and finishes. Reuse them in proposals and on your profile to speed contracting.


Disclaimer
The insights in this article are provided for informational purposes only. They are not exhaustive standards or guarantees of selection in clinical research procurement. Freelance Clinical does not provide legal, regulatory, or quality certification services. Vendors are responsible for ensuring compliance with applicable laws, regulations, and sponsor requirements. References to external companies and examples are for illustration only and do not constitute endorsements.
FOLLOW US
Disclaimer: Freelance Clinical provides freelancer and vendor sourcing services for clinical research support. We do not claim to list or compare all available providers in the market. All information and recommendations are general in nature and do not constitute endorsements or guarantees. Clients are responsible for conducting their own due diligence before engaging third parties. Freelance Clinical is not liable for any outcomes resulting from such engagements. This website is governed by the laws of New South Wales, Australia.
ABN: 12 671 763 643 · © 2025 Freelance Clinical Pty Ltd. All rights reserved.
