Start Global Trials Faster with This Australian Strategy
Accelerate Phase I trials with Australia’s CTN scheme. Learn how sponsors reduce costs, skip IND delays, and collect globally accepted data fast.
RJ Gumban
5/29/20257 min read
You’ve seen the headlines about phase I clinical trials in Australia. Faster start-up. No IND. Lower costs. But those are only the surface-level advantages.
Sponsors are choosing Australia to launch smarter global programs. They’re gaining speed, clarity, and FDA-ready data while avoiding regulatory delays.
If you’re planning a global medical trial, it pays to look deeper.
The real question is: why let slow systems and regulatory hurdles hold you back when a faster model already exists?
Faster Clinical Trial Start-Up in Australia Without an IND
Regulatory delays are a leading reason why phase I clinical trials fall behind schedule. In the U.S., the Investigational New Drug (IND) process demands extensive preclinical data, CMC documentation, and protocol details.
Once submitted, the United States Food and Drug Administration (US FDA) conducts a 30-day review. In many cases, that timeline stretches even longer.




Other regions, including Europe and Asia, have their own approval steps that can take weeks or months.
Australia offers a faster, more sponsor-driven route.
What Is the CTN Scheme and Why It Matters
Australia’s Clinical Trial Notification (CTN) scheme gives sponsors something rare in clinical research: speed without red tape.
Instead of going through a full regulatory review, you submit your trial protocol to a Human Research Ethics Committee (HREC). Once approved, you simply notify the Therapeutic Goods Administration (TGA). That’s it.
Why Global Sponsors Choose CTN for Phase I Trials
Once sponsors understand how the CTN scheme works, they don’t just see a faster start. They see a smarter way to run early phase studies.
With approvals often complete in under six weeks, sponsors can lock in timelines, reduce uncertainty, and begin dosing without the usual wait. This speed creates a ripple effect across the entire development program.
Sponsors are using CTN to:
Launch first-in-human studies ahead of schedule
Generate early clinical data to support global decisions
De-risk later phases by refining protocol elements early
Such agility is hard to ignore, with many sponsors noticing the impact.
Take Samumed, a California biotech firm that completed a Phase I trial for male pattern baldness, gaining protocol approval just 1.5 weeks after submission. Their collaboration with a private ethics committee sped up the process, allowing enrollment of 29 patients in two months.
OncoMed Pharmaceuticals experienced similar benefits. The US-based cancer therapeutics company utilized the CTN scheme to revise protocols and maintain enrollment across three oncology studies.
Their Australian partner quickly implemented changes and ensured continued data collection met FDA standards.
This trend extends beyond US companies; sponsors from Asia, Europe, and South America also adopt this strategy, selecting Australia to verify safety signals, assess dose ranges, and proceed without waiting for an IND application.


Phase I Trial Costs with Australia’s R&D Incentives
Running early phase trials in Australia is not only faster, it’s also significantly more cost-effective.
The average cost of a Phase I trial in Australia ranges from USD 1.2 million to USD 2.5 million, compared to USD 1.4 million to USD 6.6 million in the United States. That baseline savings alone gives sponsors more flexibility and budget control from the start.
The financial advantage grows even stronger with Australia’s R&D Tax Incentive, which provides a tax offset for eligible clinical trial expenses.
Companies with annual turnover below AUD 20 million can claim a refundable offset equal to their corporate tax rate plus an 18.5% premium.
Companies above AUD 20 million receive a non-refundable offset based on how much of their total spending goes to R&D:
The first 2% of eligible R&D earns an offset of corporate rate plus 8.5%
Any additional spend beyond that earns corporate rate plus 16.5%
These offsets apply to eligible R&D activities conducted from 1 July 2021 onward, administered by the Australian Taxation Office and the Department of Industry, Science, and Resources.
With these incentives in place, sponsors are able to stretch their trial budgets further. That often means increasing patient enrollment, broadening study scope, or reinvesting in follow-up trials, all while maintaining high scientific and regulatory standards.
Here’s how several companies have benefited:
Nacuity Pharmaceuticals saved over 12 months in time and costs by relocating operations and leveraging local R&D support services.
Clarity Pharmaceuticals secured an AUD 11.1 million refund under the R&D Tax Incentive to accelerate its oncology programs.
Paradigm Biopharmaceuticals received AUD 6.3 million to fund Phase III work in osteoarthritis, strengthening its cash reserves and clinical momentum.


How to Start a Clinical Trial in Australia
Getting started in Australia isn’t complicated, but it does require the right local support and a clear plan. The CTN scheme puts timelines back in the sponsor’s hands, but only if each step is handled with precision and care.
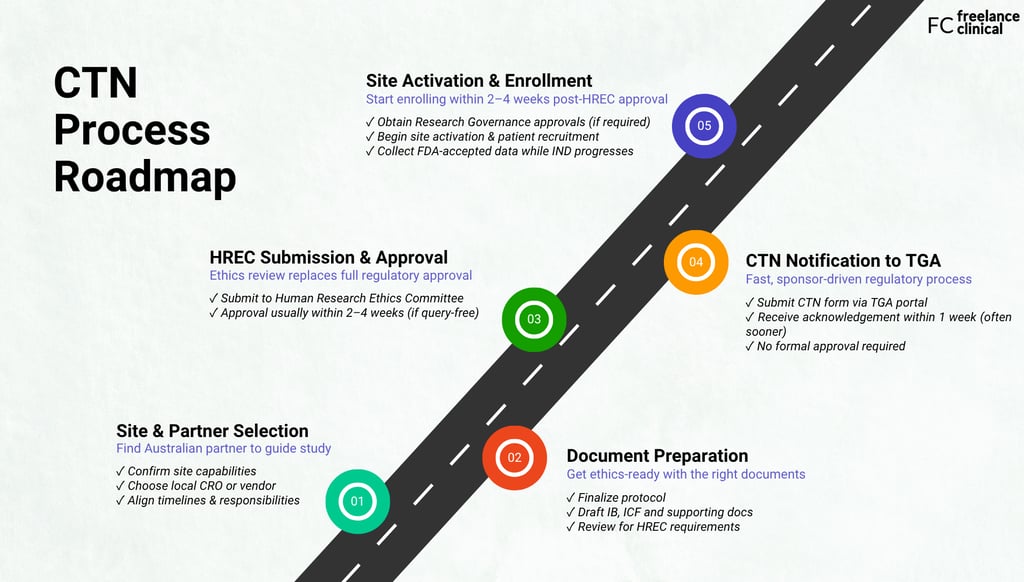
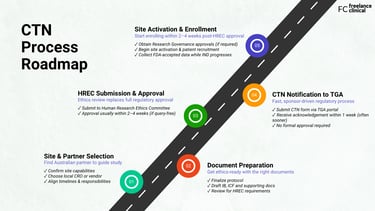
Here’s what sponsors need to do to launch their early phase trial successfully:
FDA campus in Silverspring, Maryland
Key takeaway: The CTN scheme lets sponsors launch Phase I trials in Australia within weeks while preparing their U.S. IND, gaining global-quality data without delays.
No clinical hold. No long review queues. Just a streamlined path that puts timelines back in your control.
For many early phase studies, you can move from protocol to first patient in within 4 to 6 weeks. That kind of speed shifts what’s possible, especially when your U.S. IND is still in progress.
With the CTN scheme, sponsors do not have to wait for the FDA before getting started. You can generate early data in Australia while your U.S. application moves forward in parallel.
It’s not just about moving faster. Data collected through the CTN pathway is accepted by the US FDA, the European Medicines Agency (EMA), and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA).
This means you often avoid repeating Phase I trials in other regions. You save time, reduce costs, and build global momentum while staying aligned with high regulatory standards.
Source: Forbes
Step 1: Work with a partner who knows the CTN process
Before anything else, sponsors need a qualified partner in Australia. This isn’t just for compliance. It’s to navigate faster start-up, ethics approvals, and trial site coordination.
Freelance Clinical helps sponsors manage every step, from resourcing to vendor search and selection, all tailored to fit study timelines.
Step 2: Finalize protocol and supporting documents
Prepare your trial protocol, investigator brochure, informed consent forms, and safety data. The HREC will review these documents, so clarity and scientific merit are crucial from the outset.
Step 3: Submit to an HREC for ethics review
HRECs review both the scientific and ethical elements of your medical trial. This is the core approval step. Many private HRECs can review within 2 to 4 weeks, helping you avoid unnecessary bottlenecks.
Step 4: Notify the Therapeutic Goods Administration
After HREC approval, sponsors submit a CTN to the TGA. No lengthy review. No clinical hold. Just notification. This streamlined step keeps the regulatory burden low and the study on schedule.
Step 5: Activate your sites and enroll
Once TGA notification is complete, you can begin activating sites and enrolling patients, depending on whether Research Governance Office (RGO) approval is required. Choosing the right sites early can make all the difference.
Freelance Clinical can help you identify high-performing sites aligned with your timeline, budget, and protocol. With the right setup, many early phase trials move from protocol to first patient within 4 to 6 weeks.
Make Australia Your Early Phase Advantage
Australia is more than a fast-track destination. It’s a strategic launchpad for global trials.
With the Clinical Trial Notification scheme, generous R&D tax incentives, and internationally accepted data, sponsors gain the speed, clarity, and flexibility they need to make better decisions early.
Those first few months can set the tone for your entire program. A smart start now can reduce risks, strengthen submissions, and accelerate time to market.
Freelance Clinical helps sponsors maximize the full potential of Australia’s ecosystem. From ethics approvals to site activation, our experts streamline the process and support your early phase medical trial goals.
Ready to move faster with fewer delays? Submit a request here or email us at info@freelanceclinical.com.
Disclaimer
This article is intended for informational purposes only and does not constitute legal, regulatory, or medical advice. The content reflects publicly available guidance from the Therapeutic Goods Administration (TGA) and other cited sources. Readers should consult qualified regulatory professionals or relevant authorities before making decisions related to clinical trial planning, approvals, or submissions. While efforts have been made to ensure accuracy, Freelance Clinical does not guarantee the completeness or current applicability of the information presented.
Key takeaway: CTN enables sponsors to start earlier, adapt faster, and deliver globally accepted data with fewer regulatory delays.
Key takeaway: Sponsors can reduce early phase trial costs by over 40% in Australia by combining lower operational expenses with robust R&D tax offsets.
FOLLOW US
Disclaimer: Freelance Clinical provides freelancer and vendor sourcing services for clinical research support. We do not claim to list or compare all available providers in the market. All information and recommendations are general in nature and do not constitute endorsements or guarantees. Clients are responsible for conducting their own due diligence before engaging third parties. Freelance Clinical is not liable for any outcomes resulting from such engagements. This website is governed by the laws of New South Wales, Australia.
ABN: 12 671 763 643 · © 2025 Freelance Clinical Pty Ltd. All rights reserved.
